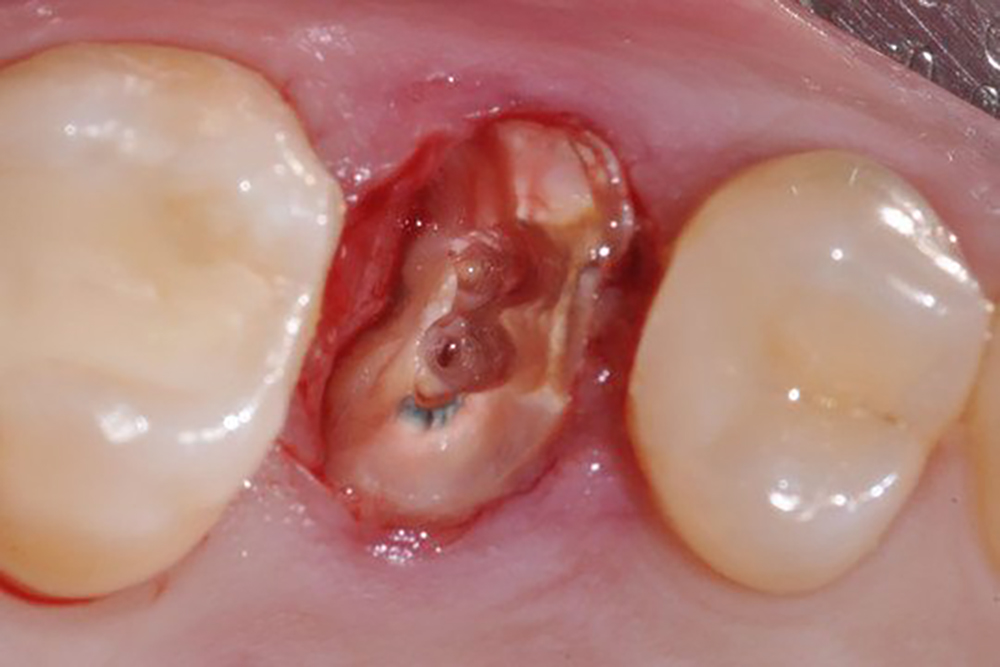
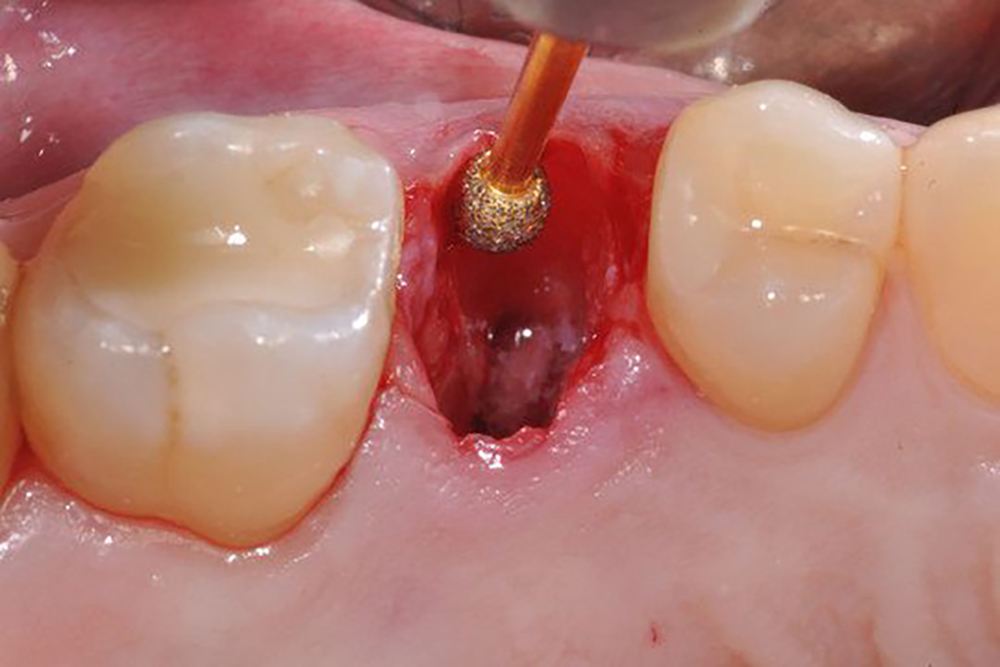
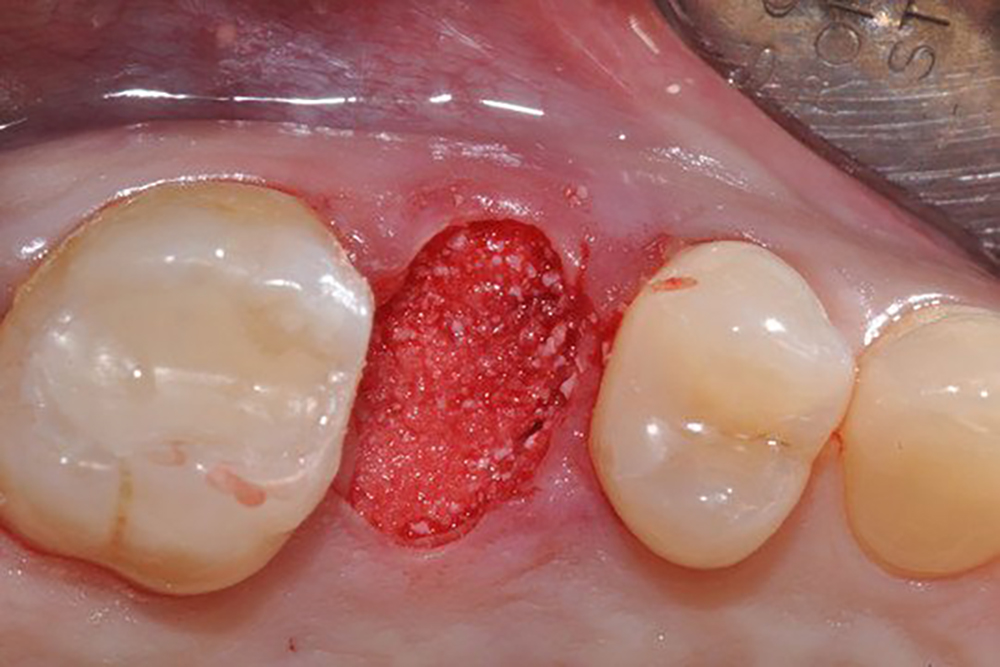
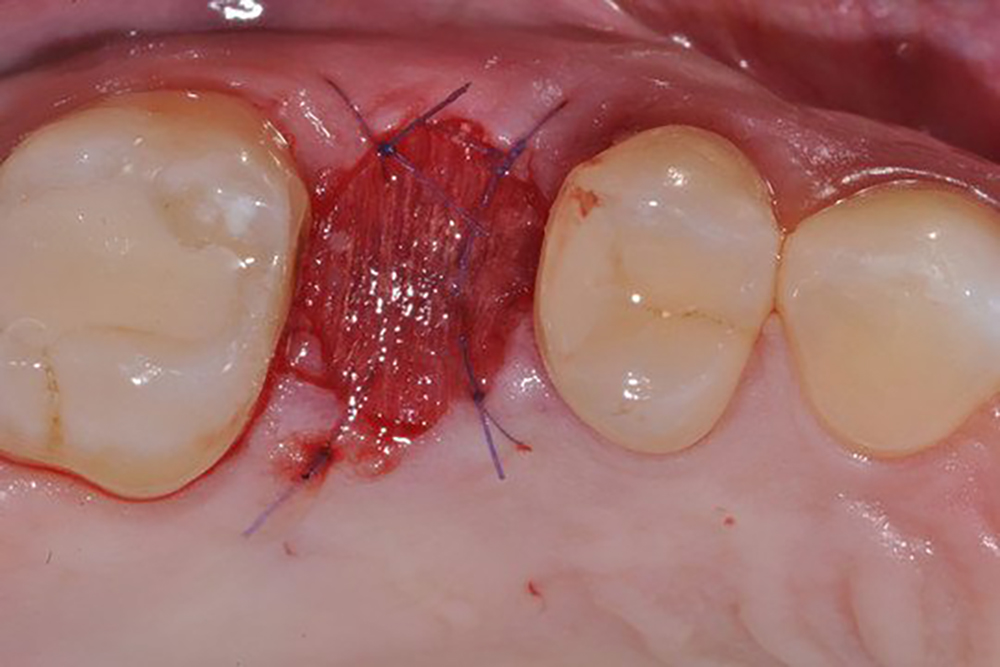
תיאור
Description
Leading clinicians rely on Geistlich Mucograft Seal®:
- Minimal invasion¹ ²
- No harvest site morbidity¹
- Creates predictable soft-tissue dimensions for later soft-tissue management and allows for flexible timing of implant placement¹
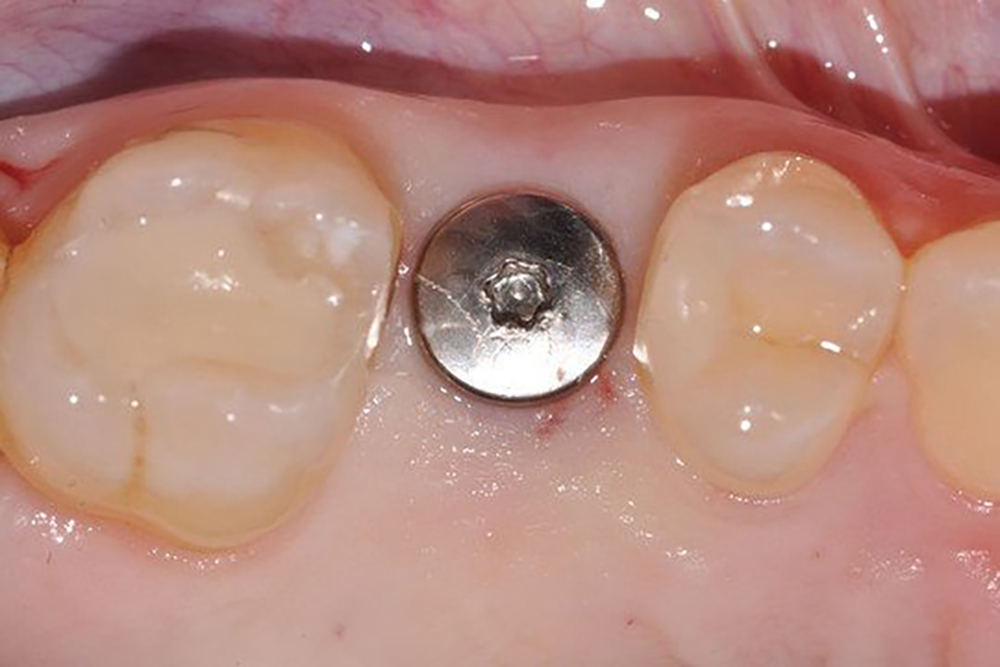
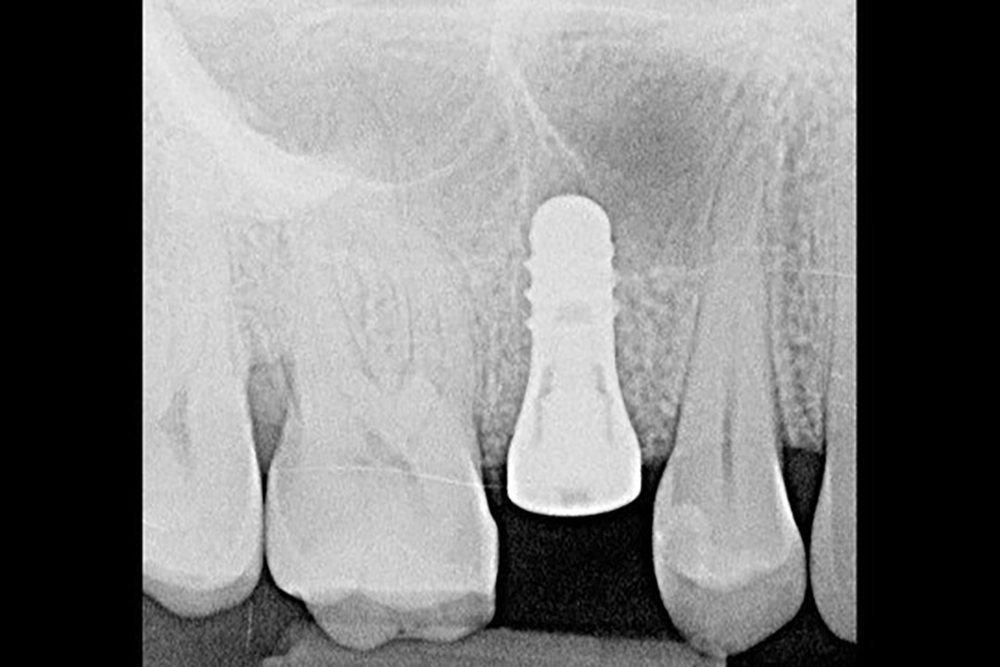
- Early implant placement possible 8-10 weeks after extraction¹
- Reduced surgical chair-time compared with autogenous grafts¹
- Faster soft-tissue wound healing compared with autogenous grafts³
- Natural soft-tissue colour and texture match¹ ³
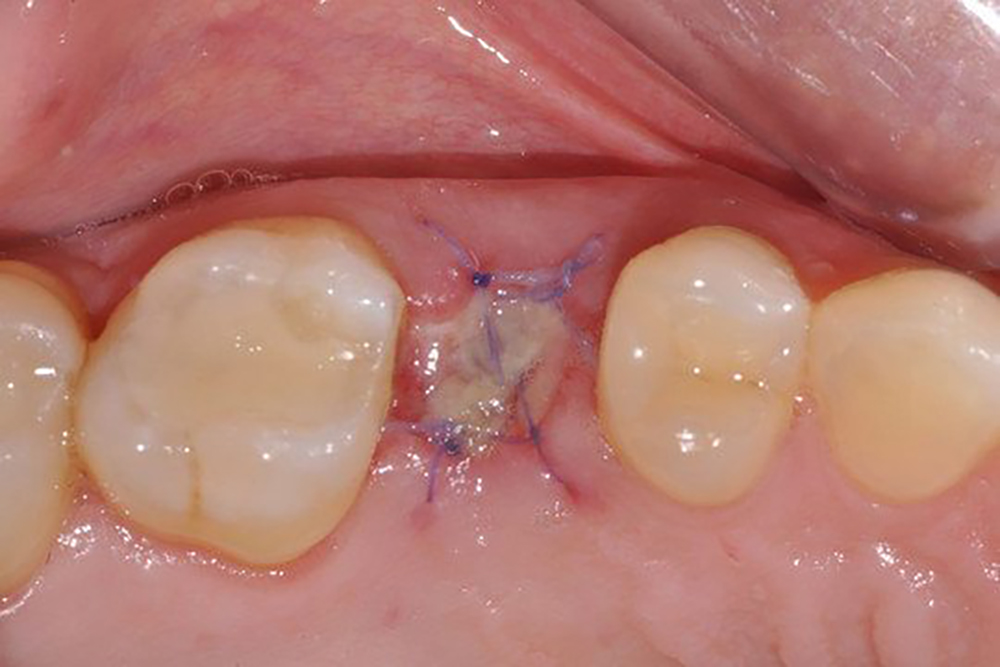
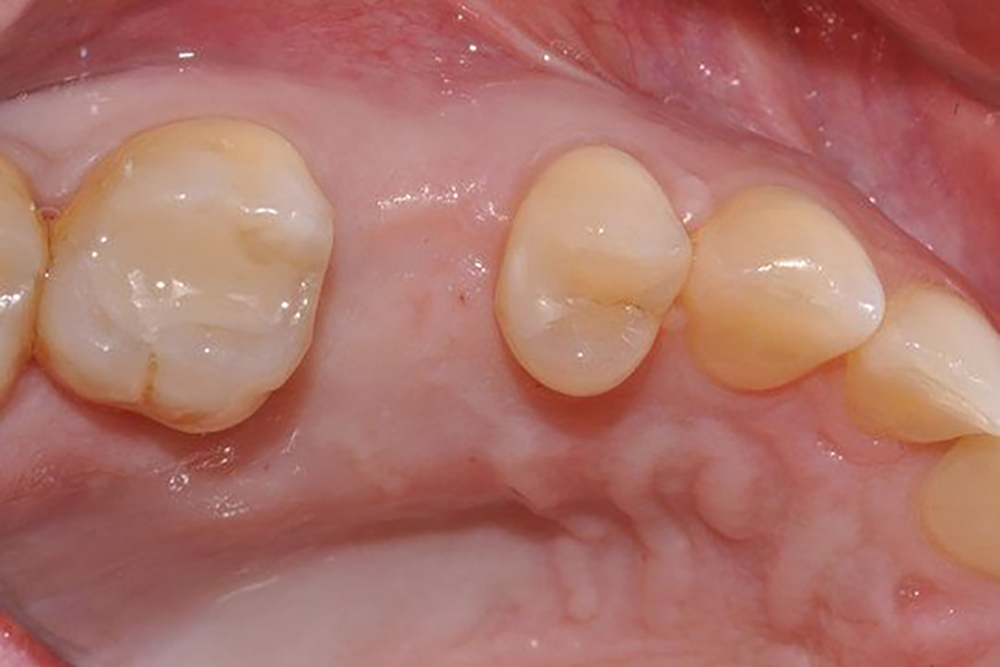
Geistlich Mucograft® Seal consists of two structures: the compact structure provides stability while allowing open healing; the spongy structure supports blood clot stabilisation and ingrowth of soft-tissue cells.
References:
1. Geistlich Mucograft® Seal Advisory Board Meeting Report, 2013. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
2. Jung R, et al.: J. Clin Periodontol 2013; 40(1): 90-98. (clinical study)
3. Thoma D, et al.: J. Clin Periodontol 2012; 39: 157-65. (clinical study)